Blood Biomarkers for Alzheimer’s Disease : Early Diagnosis
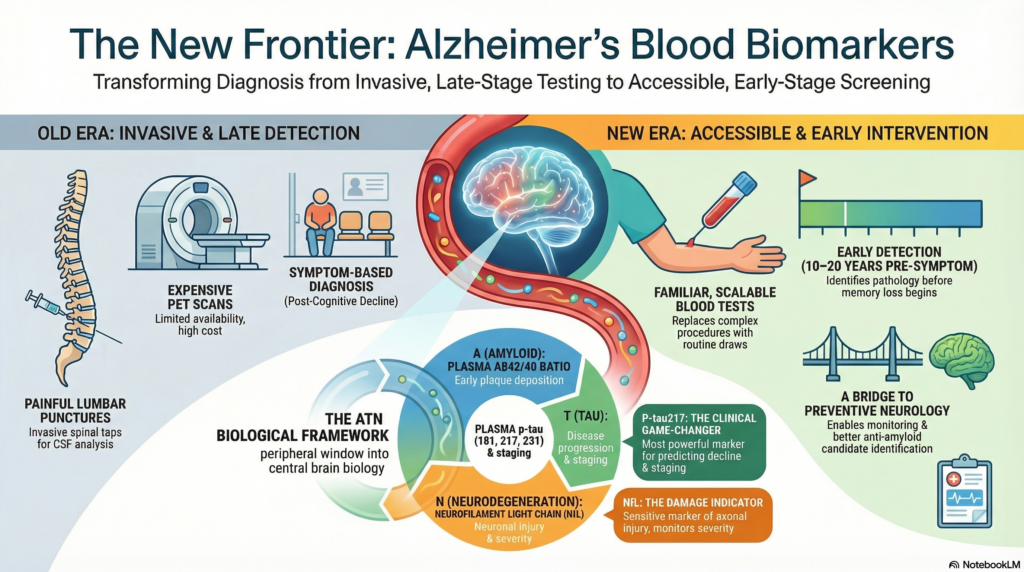 For most of modern medical history, Alzheimer’s disease could only be diagnosed with confidence after death. Even during life, certainty required expensive imaging or invasive cerebrospinal fluid (CSF) testing. This practical barrier shaped everything—from delayed diagnosis to failed drug trials.
For most of modern medical history, Alzheimer’s disease could only be diagnosed with confidence after death. Even during life, certainty required expensive imaging or invasive cerebrospinal fluid (CSF) testing. This practical barrier shaped everything—from delayed diagnosis to failed drug trials.
That story is now changing.
Blood biomarkers for Alzheimer’s disease represent one of the most important breakthroughs in neuroscience and clinical medicine in the last decade. They bring Alzheimer’s diagnosis out of the PET scanner and lumbar puncture room and into something far more familiar: a blood test.
Quietly, but decisively, the field is shifting.
Why blood biomarkers matter
Alzheimer’s disease begins 10–20 years before symptoms. Yet traditional diagnosis has relied on memory complaints, neuropsychological testing, and imaging performed far too late in the disease course.
Blood biomarkers solve several long-standing problems:
-
Enable early and preclinical detection
-
Reduce dependence on expensive PET imaging
-
Avoid invasive lumbar punctures
-
Allow large-scale screening and follow-up
-
Align directly with the ATN biological framework
In short, they allow Alzheimer’s disease to be treated like other chronic medical illnesses—detected early, monitored longitudinally, and intervened upon rationally.
How blood biomarkers fit into the ATN framework
Blood biomarkers map neatly onto the ATN model:
-
A (Amyloid pathology) → plasma Aβ42/40 ratio
-
T (Tau pathology) → plasma phosphorylated tau (p-tau)
-
N (Neurodegeneration) → plasma neurofilament light chain (NfL)
This is not coincidence. Blood biomarkers are essentially a peripheral window into central brain biology.
Amyloid blood biomarkers (A)
Plasma Aβ42/Aβ40 ratio
Amyloid-β peptides are produced in everyone. What matters is the relative balance, not the absolute level.
-
In Alzheimer’s disease, Aβ42 preferentially deposits in plaques
-
This leads to a reduced Aβ42/Aβ40 ratio in blood
Modern ultra-sensitive assays (Simoa, mass spectrometry) now show strong correlation between plasma amyloid ratios and amyloid PET positivity.
Clinical meaning:
A reduced ratio suggests early Alzheimer’s pathology, often before symptoms appear.
Tau blood biomarkers (T): the real game-changer
If amyloid marks disease presence, tau predicts disease progression.
Plasma phosphorylated tau (p-tau)
Among all biomarkers, plasma p-tau has emerged as the most powerful and clinically relevant.
Key variants include:
-
p-tau181
-
p-tau217
-
p-tau231
Of these, p-tau217 has shown the strongest correlation with:
-
Tau PET imaging
-
Cognitive decline
-
Disease staging
Importantly, plasma p-tau is highly specific to Alzheimer’s disease, unlike many older markers.
Clinical meaning:
Elevated plasma p-tau strongly suggests biological Alzheimer’s disease, even in mild or atypical cases.
Neurodegeneration biomarkers (N)
Neurofilament light chain (NfL)
NfL is a structural protein released into blood when axons are damaged.
-
Elevated in Alzheimer’s disease
-
Also elevated in FTD, vascular dementia, ALS, MS, and traumatic brain injury
This makes NfL sensitive but not specific.
Clinical meaning:
NfL tells us that neurons are being injured—but not why.
In combination with amyloid and tau markers, it helps stage disease severity and monitor progression.
Other emerging blood biomarkers
Glial fibrillary acidic protein (GFAP)
GFAP reflects astrocytic activation and neuroinflammation.
-
Often elevated early, sometimes even before tau
-
May act as an early “stress signal” of amyloid pathology
Inflammatory and synaptic markers
Ongoing research is exploring:
-
Complement proteins
-
Cytokine signatures
-
Synaptic proteins (e.g., neurogranin)
These may eventually add a fourth dimension to ATN-based models.
Clinical applications today
Blood biomarkers are already reshaping practice in subtle but important ways:
-
Screening patients with subjective cognitive decline
-
Differentiating Alzheimer’s disease from depression-related cognitive symptoms
-
Identifying appropriate candidates for anti-amyloid therapies
-
Reducing unnecessary imaging
-
Tracking disease progression and treatment response
In research settings, blood biomarkers now act as gatekeepers, deciding who proceeds to PET imaging or clinical trials.
Important limitations (and healthy skepticism)
Blood biomarkers are powerful—but not magical.
Key caveats:
-
They do not replace clinical judgment
-
Cut-offs vary across assays and populations
-
Comorbidities (renal disease, inflammation) may influence levels
-
Ethical implications of preclinical diagnosis remain unresolved
A blood test should never be interpreted in isolation. Context remains king.
What this means for the future
We are approaching a world where:
-
Alzheimer’s disease is diagnosed biologically, early, and accurately
-
Dementia becomes a late-stage complication, not the starting point
-
Preventive neurology becomes realistic, not speculative
-
Treatment trials target the right patients at the right time
Blood biomarkers are the bridge between neuroscience and real-world medicine.
Closing reflections
The most radical idea in modern Alzheimer’s care is not a new drug—it is early truth.
Blood biomarkers bring that truth closer, cheaper, and earlier than ever before. They allow us to move from reactive care to anticipatory medicine, from late labels to early understanding.
Alzheimer’s disease has always been a biological process.
We are finally learning how to listen—without opening the skull.
Dr. Srinivas Rajkumar T, MD (AIIMS), DNB, MBA (BITS Pilani)
Consultant Psychiatrist & Neurofeedback Specialist
Mind & Memory Clinic, Apollo Clinic Velachery (Opp. Phoenix Mall)
✉ srinivasaiims@gmail.com 📞 +91-8595155808